- Home
- Private: Products
- Drug Coated Balloon
- Lutonix 035
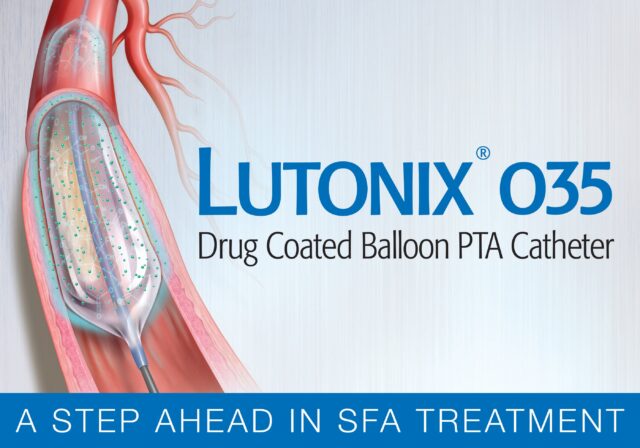
Lutonix 035
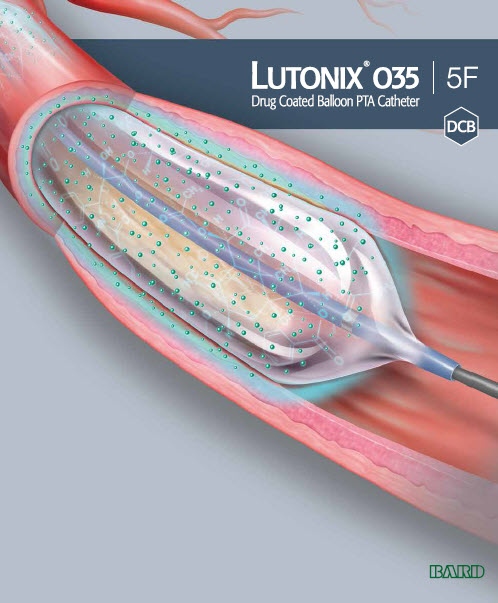
Lutonix® 035 Drug Coated Balloon PTA Catheter | 5F
Maximized Efficacy, Indisputable Safety
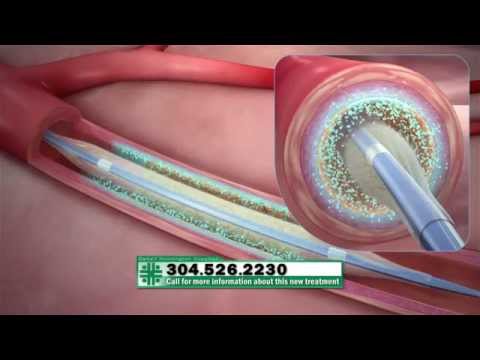
The Lutonix® DCB catheter is a drug-coated balloon catheter that delivers paclitaxel to the arterial wall in a single, short inflation. Paclitaxel is an anti-proliferative drug commonly used to prevent arterial restenosis. The Lutonix® DCB’s highly efficient formulation of paclitaxel and carriers, polysorbate and sorbitol, allows Lutonix® DCB catheter to deliver a therapeutic dose to the artery wall, while keeping the dose of paclitaxel on the balloon as low as possible.
Lowest Profile DCB*
The Lutonix® 035 DCB catheter is the only DCB with all offered sizes 5F, making it the lowest profile DCB on the U.S. market.* This platform is designed to minimize the size of the access site, while maintaining an ease-of-use characteristic similar to PTA. Lutonix® 035 DCB 5F is available in the following sizes:
- Lengths: 40, 60, 80, 100, 120, 150 mm
- Diameter:4-6 mm up to 150 mm, 7 mm up to 60 mm
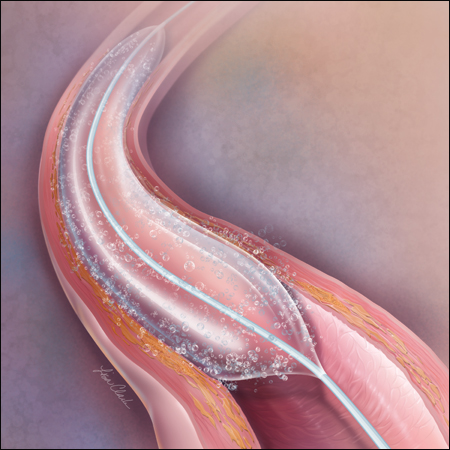
Increased Procedure Efficiency with GeoAlign®
The Lutonix® DCB features the GeoAlign® Marking System. GeoAlign® Markers serve as a simple-to-use, non-radiopaque ruler on the catheter shaft. The GeoAlign® System is featured on both the Ultraverse® 035 PTA Catheter and Lutonix® 035 DCB. The GeoAlign® System is designed to facilitate repeatable catheter alignment at the lesion and increase procedure efficiency by minimizing fluoroscopy exposure.**
-
- Lutonix® 035 – IFU
- Lutonix® 035 – Global Brochure (with sizes and specifications)
- Lutonix® 035 – 5F Brochure
- Lutonix® 035 – GeoAlign® Marking System Brochure
- Lutonix® 035 – LEVANT 2 Brochure
*Lutonix® offered sizes as of March 2016: lengths of 40, 60, 80, 100, 120, 150 mm, diameters of 4-6 mm up to 150 mm, 7 mm up to 60 mm.
**GeoAlign® Markers are not a replacement for fluoroscopy. When the catheter is exposed to the vascular system, the location of the balloon should be confirmed while under high quality fluoroscopic observation. Animal study (repeat PTA in swine artery) was performed by 3 physicians who tested the Lutonix® 035 DCB (no drug) and the Ultraverse® 035 PTA Catheter, both with GeoAlign® Markers, to POBA with no GeoAlign® Markers (n=112, test n = 96, control n = 16). Animal data on file. Bard. Animal test results may not be indicative of clinical performance. Different test methods may yield different results.
The Lutonix® 035 Drug Coated Balloon PTA catheter is indicated for percutaneous transluminal angioplasty, after appropriate vessel preparation, of de novo, restenotic, or in-stent restenotic lesions up to 300mm in length in native superficial femoral or popliteal arteries with reference vessel diameters of 4-7mm.
Please consult Instructions for Use under Resources for product indications for use, contraindications, warnings, precautions, complications, adverse events and detailed safety information.